Strategic interactions
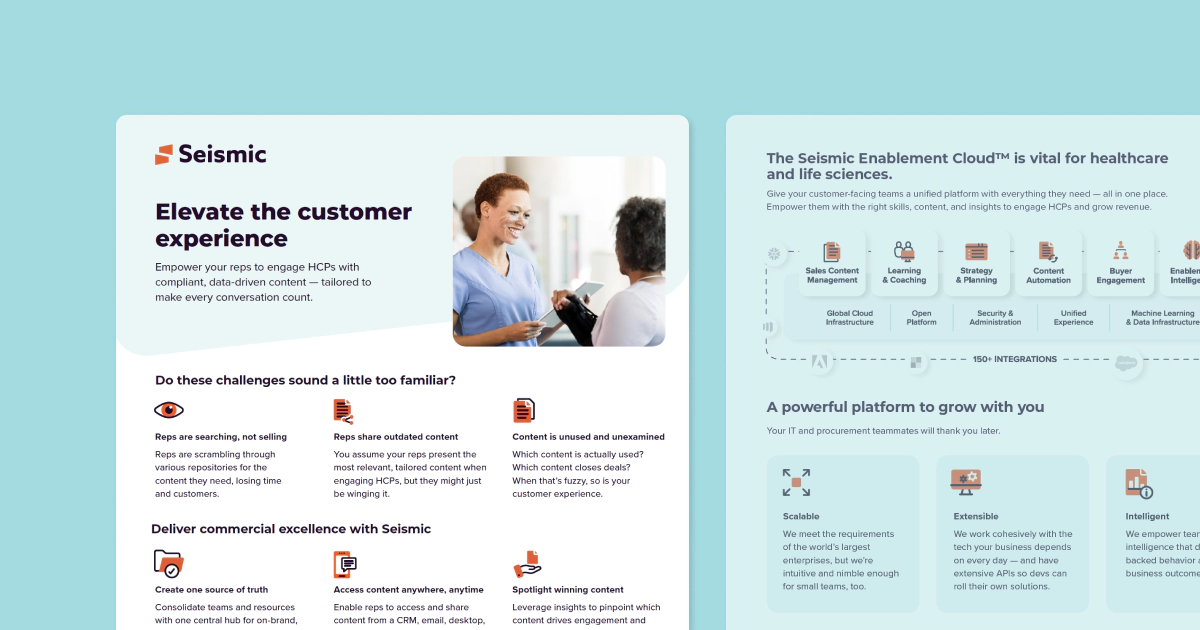
Align marketing and sales with intuitive tools that surface targeted content and insights, allowing reps to spend more time engaging with HCPs.
Impactful benefits
Compliant content
Engage HCPs and eliminate risk with on-brand, compliant content.
Embedded in workflows
Share rich content from a CRM, email, desktop, or mobile device.
Timely data
Enable reps to make informed decisions fast with real-time analytics.
The modern sales experience
Compliance
Govern content, ensuring that reps only share accurate and compliant information, data, and materials with customers.
Mobile-first access
Provide relevant documentation via a user-friendly mobile interface to enable reps onsite with customers — even offline.
Performance data
Refine conversations with AI-driven content recommendations and see what content is getting used and shared.
Just-in-time learning
Bring reps up to speed on new products and terminology in weeks, not months, and increase their knowledge retention.
Seamless integrations
Connect Seismic with sales and marketing technologies that teams rely on such as Salesforce, Adobe, Veeva Vault, and SharePoint. Access content with the click of a button from a mobile device, CRM, or email.
View Seismic Exchange®Speak with our team!
Make way for more wins with Seismic
Real-world success

Content automation saved Illumina 6,650+ hours